Last Thursday, the cardiology consultant argued that dividing heart failure symptoms into left sided and right sided did not make sense. After all, if the left pump is failing, there will be backwards and forwards failure. The forwards failure will reduce venous return, which will reduce the right ventricular output by reducing preload. The backwards failure will increase the pulmonary pressure, and will eventually reduce the right ventricular output by increasing afterload. It is therefore impossible to have purely isolated symptoms of univentricular heart failure, and making the distinction between left and right sided failure clinically is meaningless.
This was shocking to me and the students. We had been taught so much about left sided vs right sided failure. I decided to go back to basic science and evidence to see if I could work it out for myself.
What is the role of the right ventricle?
In 1943 and seemingly oblivious to some sort of war going on, Starr et al ablated the RV in open chest dogs with “a red hot soldering iron,” and found little alteration in systemic circulation or venous pressure.
Starr I, Jeffers WA, Meade RH. The absence of conspicuous increments of venous pressure after severe damage to the RV of the dog, with discussion of the relation between clinical congestive heart failure and heart disease. Am Heart J. 1943;26:291-301.
At the highly misogynist time, everyone thought that this meant the right ventricle was little more than a pretty bride to the hunky husband of the left ventricle, which was the dominant pump determining cardiac output.
And so it remained, until further studies showed that this may be true in normal health, but the moment pulmonary pressures increase the performance of the right ventricle falls off. This situation arises in cor pulmonale, acute PE (which can be thought of as acute cor pulmonale)…and significant left ventricular failure.
Brooks H, Kirk ES, Vokonas PS, Urschel CW, Sonnenblick EH. Performance of the right ventricle under stress: relation to right coronary flow. J Clin Invest. 1971;50:2176-83
More recently, it was shown that having a right ventricular infarct on top of your (usually inferior) left sided infarct was associated with a worsened mortality and morbidity.
Zehender M, Kasper W, Kauder E, Schonthaler M, Geibel A, Olschewski M, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993;328:981-8.
Interestingly, they found that having a right ventricular infarct on top of your LV infarct led to worsened hypotension, which is a classical ‘left sided’ symptom from forwards failure. This study noted this effect was independent of the size of the left sided infarct. The findings have been replicated where shock was more common in those patients who had right sided failure. So, the right ventricle’s function is important, especially when the left ventricle is impaired. This is consistent with our consultant’s message at the start that the failure of one ventricle invariably messes up the other.
LV function is also a direct determinant of RV ejection, via the effects of LV function on the contraction of the interventricular septum and the wringing action of the LV.
In the same article, we can see how impaired RV function can lead to a dilated and hypertrophied right ventricle, which in turn can shift the septum towards the left and impair LV filling.
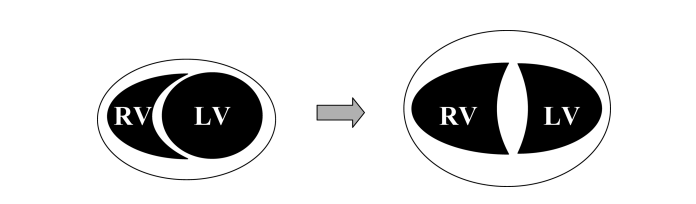
RV hypertrophy occurs as a response to increased wall stress. The growing RV forces the IV septum into the left ventricle, as the pericardium prevents the outwards growth of the right ventricle.
Does this answer the question of whether or not the forwards/backwards failure means you always have the same symptoms anyway?
Although we can see that failure of one ventricle leads to failure of the other, this is not the same as saying there is no clinical distinction between the two. I’m still to be convinced that there is no role at all for distinguishing the two types of failure clinically. I struggled to find any sources which suggest there is no value in distinguishing the two clinically, and that both must invariably exist together. That could be because it’s hard to search for a negative i.e. pages which do not state left and right sided symptoms separately. I still feel that isolated left/right ventricular failure is a real thing (which may progress to biventricular failure) but will keep open minded about this and be ready to claim I blogged about it first if and when the death of left/right failure as a classification becomes mainstream.